Figaro offers a wide range of gas sensor products for the detection of various gases, from explosive gases such as propane, toxic gases such as carbon monoxide, to air quality sensors for volatile organic compounds (VOCs) that are responsible for sick-house syndrome. Figaro offers a diverse portfolio of sensor technologies that can be matched to the unique requirements of each application.
Operation Principle of Short-circuit Current type Proton Conductor CO Sensor
Figaro TGS5xxx series is a proton conductor carbon monoxide (CO) sensor that utilizes short-circuit current as a sensor signal. The basic components of the gas sensing layer are a working electrode, a counter electrode, and a proton-conducting membrane in between them.
When the sensor is placed in clean air without connecting the working electrode to the counter electrode with an external wiring, i.e., under open-circuit condition, the electrochemical reaction (1) takes place on the working electrode and the counter electrode, respectively, resulting in the equilibrium potential (E1) at both electrodes.
2H+ + 1/2O2 + 2e- ⇄ H2O ...(1)
In the presence of CO mixed with air under the open-circuit condition, anodic oxidation of CO (2) and cathodic reduction of oxygen (3) proceed on the working electrode simultaneously at the same rate, forming a local cell.
CO + H2O → CO2 + 2H+ + 2e- ...(2)
2H+ + 1/2O2 + 2e- → H2O ...(3)
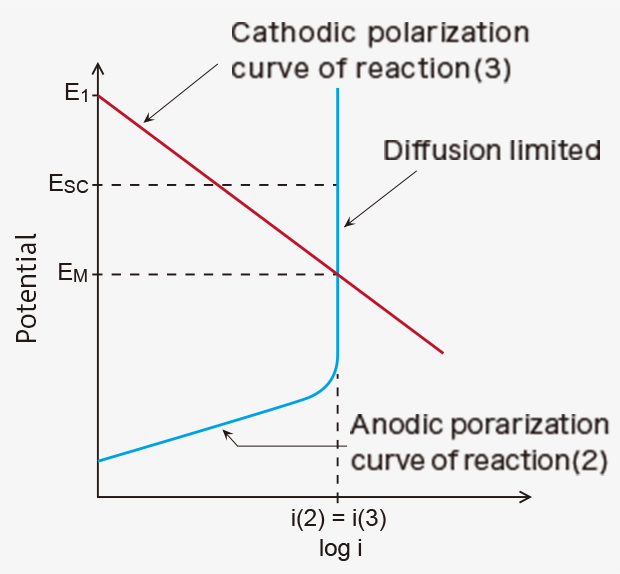
As a result, the potential of the working electrode changes from E1 to the mixed potential (EM), at which anodic current i(2) and cathodic current i(3) are equal in absolute value in a steady state (Figure 1). The volume of CO reaching the working electrode is limited by a diffusion control system, e.g., a gas diffusion control stainless film as used in the TGS5xxx series sensors, giving a diffusion limiting current (Figure 1), which is linearly proportional to CO concentration. Since only the reaction (1) takes place on the counter electrode, the potential of the counter electrode remains at E1. Thus, the sensing signal of this potentiometric (mixed-potential type) sensor can be given by EM - E1, which is proportional to the logarithm of CO concentration in air.
When the working electrode is electrically connected to the counter electrode with an external wiring to make a short-circuit condition in the presence of CO in air, the potentials of the two electrodes shift to the same value (Esc) in between E1 and EM, as shown in Figure 1. Since the potential of the working electrode changes in the direction the reaction (3) is decelerated, fewer protons are consumed on the working electrode. As a result, excess protons will transfer from the working electrode toward the counter electrode through the proton-conducting membrane. The number of the excess protons is proportional to CO concentration in air. And they are consumed by the reaction (3) on the counter electrode. This process is accompanied by a flow of the equivalent electrons from the working electrode to the counter electrode through an external wiring as a short-circuit current (sensor output current), which is also linearly proportional to CO concentration (Figures 2).
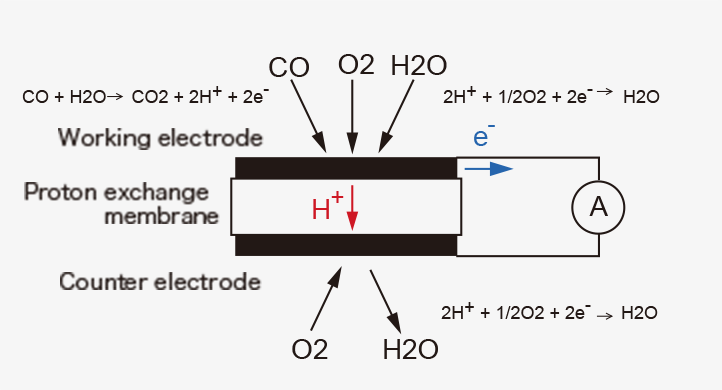
When the sensor returns to clean air, only the reaction (1) takes place on the working electrode, and current does not flow through the wiring because a potential difference between the two electrodes vanishes. Thus, the sensor shows reversible amperometric responses to CO.
Linear relationship between CO concentrations and sensor output currents makes this short-circuit current type CO sensor more accurate when compared to potentiometric CO sensors, the response of which is proportional to the logarithm of CO concentration in air. By calibrating the sensor output current with a known CO concentration, the sensor can be used for quantitative measurements of CO concentrations.
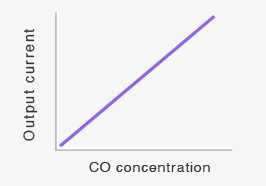
Short-circuit current (sensor output current) flowing across the external circuit will be proportional to gas concentration (see Equation 4 and Figure 3).
I = F × (A/σ) × D × C × n ...(4)
where:
I: Sensor output current
F: Faraday constant
A: Pinhole area of diffusion film
σ: Thickness of diffusion film
D: Gas diffusion coefficient
C: Gas concentration
n: Number of reaction electrons
Features
Unlike conventional dry batteries, there is no consumption of active solid or liquid materials or electrodes of the sensor. This ensures this sensor’s excellent long-term stability and maintenance-free operation for a long period. This sensor works without a heater and produces self-generating output current, making it ideal for battery-operated CO detection equipment.
Reference:
Norio Miura, Hiroshi Kato, Noboru Yamazoe, Tetsuro Seiyama, A Proton Conductor Gas Sensor Operative at Ordinary Temperature, Denki Kagaku, 50, No. 10, 858-859 (1982)
Norio Miura, Hiroshi Kato, Yoshihiro Ozawa, Noboru Yamazoe, Tetsuro Seiyama, Amperometric Gas Sensor Using Solid State Proton Conductor Sensitive to Hydrogen in Air at Room Temperature, Chem. Letters, 1905-1908, (1984)
Norio Miura, Hiroshi Kato, Noboru Yamazoe, Tetsuro Seiyama, Amperometric Proton-Conductor Sensor for Detecting Hydrogen and Carbon Monoxide at Room Temperature, ACS Symposium Series 309, Fundamentals and Applications of Chemical Sensors, 12, 203-214 (1986)

